MADISON, Wis., Oct. 6, 2020 /PRNewswire/ — Atrility Medical today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the AtriAmp™ Device. The AtriAmp facilitates more efficient monitoring and treatment of atrial arrhythmias, a common side-effect of many heart surgeries. Existing hospital medical equipment is utilized by the AtriAmp to provide a continuous display of an intracardiac atrial electrogram, the ideal tool in diagnosing atrial arrhythmias.
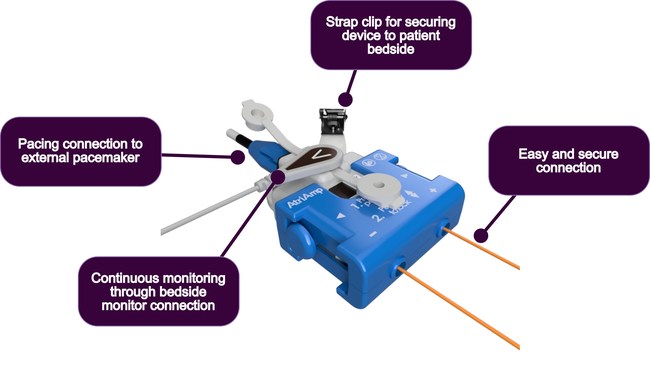
“This technology is very helpful for the physician and staff in the intensive care units as earlier recognition of the arrhythmia substrate leads to better and more defined means of arrhythmia management,” said Vincent C. Thomas, M.D., Pediatric Cardiologist and Electrophysiologist, formerly at University of Nebraska Medical Center. “Having this information present at the bedside gives a quick and easily manageable way to interpret the patient’s rhythm without the need of waiting for an ECG technologist. With this simple concept, I believe that the way we evaluate and manage post-operative arrhythmias will be disrupted and changed for the better.”
Atrial Arrhythmias are abnormal heart rhythms which originate in the atrial chambers of the heart. Along with being difficult to detect, post-operative atrial arrhythmias occur frequently and can place an immense burden on the patient and hospital.1,2 While many arrhythmias may be transient and produce little morbidity, some cause severe negative outcomes such as hypotension or congestive heart failure. These outcomes may lengthen the period of postoperative hospitalization, necessitate posthospital medications, and increase the total cost of medical care.3
“We invented the AtriAmp to overcome the challenges of timely and accurate rhythm identification immediately after heart surgery in my most vulnerable patients. Accurate and rapid diagnosis can be lifesaving in these patients,” said Nicholas Von Bergen, M.D., Associate Clinical Professor of Pediatrics & Director of the Pediatric Electrophysiology Lab at American Family Children’s Hospital, University of Wisconsin – Madison and principal inventor of the device. “With the AtriAmp, we can now provide the highest quality signal for rhythm identification on the most accessible location, the bedside monitor. At the same time, if necessary, we can also use the AtriAmp to facilitate atrial pacing. In this way, we can improve speed, accuracy and safety for better outcomes of our post-operative patients.”
About Atrility Medical
Atrility Medical is a University of Wisconsin – Madison spinoff company, with the AtriAmp as its first FDA approved product. Atrility continues to develop products through its established UW – Madison innovation pipelines, primarily for the cardiac and pediatric markets.
[1] Yadava, M., Andrew B Hughey and T. Crawford. “Postoperative Atrial Fibrillation: Incidence, Mechanisms, and Clinical Correlates.” Heart failure clinics 12 2 (2016): 299-308.
[2] McRae, Marion E., Alice Chan, and Flerida Imperial-Perez. “Cardiac surgical nurses’ use of atrial electrograms to improve diagnosis of arrhythmia.” American journal of critical care: an official publication, American Association of Critical-Care Nurses 19 2 (2010): 124-33; quiz 134.
[3] Creswell, L. L., R. Schuessler, M. Rosenbloom and J. Cox. “Hazards of postoperative atrial arrhythmias.” The Annals of thoracic surgery 56 3 (1993): 539-49.The names of actual companies and products mentioned herein may be the trademarks of their respective owners.
Media contact:
Pete Lukszys
255730@email4pr.com
SOURCE Atrility Medical

