MORRISTOWN, N.J., May 11, 2021 /PRNewswire/ — PLT Health Solutions, Inc. announced that ceratiq® Phytoceramides has been approved for sale in the Republic of Korea. A multi-year effort involving scientific and regulatory collaboration between PLT Health Solutions, ingredient innovator The Robertet Group (Grasse, France) and Korean distributor NOVAREX Co Ltd (Cheongju, Chungbuk, Korea) was behind the ingredient approval. ceratiq is a ceramide-rich natural ingredient for dietary supplements and cosmetics. It offers a range of benefits that are associated with youthful skin including improved overall skin health, reduced appearance of wrinkles, increased elasticity, suppleness and a more radiant appearance. In its Certificate of Functional Ingredient for Functional Health Foods, the claim associated with ceratiq is: “May help to maintain skin moisturizing.” The ingredient, which was named Ingredient of the Year in the Healthy Aging Category by Nutraingredients-USA in 2018, has previously been featured in leading beauty-from-within products in the USA and Europe.
According to Jay Martin, Managing Director, Global Sales at PLT Health Solutions, the approval is a significant step forward for Robertet and PLT in the effort to help consumers and product formulators connect with ceratiq’s unique science and benefits. “The South Korean – K-Beauty – market is one of the most demanding in the world and one of the most influential. As a beauty-from-within ingredient, ceratiq Phytoceramides is perfect for the proactive ‘skin first’ philosophy central to the market,” he said. “Getting approval for ceratiq in the South Korean market was a collaborative effort between the scientists and quality teams of PLT, Robertet and NOVAREX to meet the rigorous approval process. We’re proud of what we’ve accomplished and have great expectations for new brands to enter the market with this innovative ingredient,” he said.
Strong Clinical Support Builds Trust and Momentum
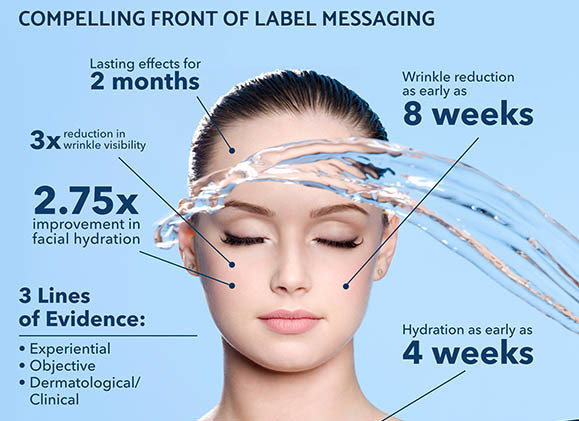
ceratiq Phytoceramides is a leading dietary supplement beauty ingredient demonstrating powerful benefits for wrinkle reduction and skin hydration.
Most recently, a double-blind, placebo-controlled study was designed to evaluate the efficacy of ceratiq on skin hydration and on reduction of wrinkle appearance (Boisnic, 2019). The subjects received either 350 mg of ceratiq or placebo for 12 weeks. In the ceratiq group, wrinkles were significantly and visibly reduced, from 8 weeks (p < 0.001), compared to the placebo. The wrinkle reduction was visible for 88% of women after 12 weeks. The results demonstrated that skin was better hydrated as early as just 4 weeks. The persistence of gains in hydration and wrinkle reduction were demonstrated for two additional months after supplementation had concluded. Compared to the placebo, ceratiq was shown to offer three times the improvement in wrinkle visibility, 2.75 times the improvement in facial hydration and five times the improvement of radiance.
According to Irene Lamour, Business Development Director at the Robertet Health & Beauty division of the Robertet Group, the recent successes of ceratiq in global markets further validates the company’s vision for a high-quality beauty-from-within ingredient. “When we set out to create ceratiq, we wanted to have a sustainably-sourced, premium quality plant-based ingredient backed by a strong body of clinical science. We believed that a nutrition-based solution could be the “new cosmetics” and that it makes sense for consumers that nutrition impacts the skin health and beauty. The age demographic for a product containing ceratiq runs from millennials to seniors, which can mean a lifetime relationship with consumers,” she said.
For more information, visit: www.plthealth.com/ceratiq.
References
- Boisnic S, Keophiphath M, Serandour AL, et al. Polar lipids from wheat extract oil improve skin damages induced by aging: Evidence from a randomized, placebo-controlled clinical trial in women and an ex vivo study on human skin explant. J Cosmet Dermatol. 2019;18(6):2027–2036. doi:10.1111/jocd.12967
Media Contact:
Mark Falconer
Sciencewerks
Voice: 407-412-9705
E-mail:
309613@email4pr.com
Company Contact:
Steve Fink
PLT Health Solutions, Inc.
Voice: 973-984-0900 x214
E-mail:
309613@email4pr.com
SOURCE PLT Health Solutions